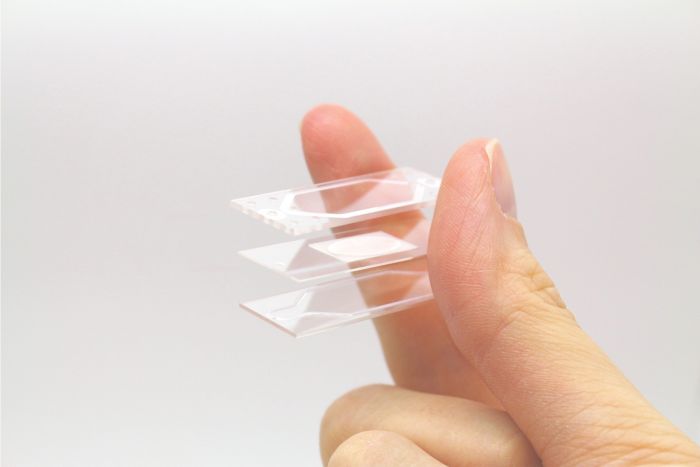
Organ-on-a-chip membrane layer
per pack of 12
Pack of 12 middle layers, to be placed in between top and bottom organ-on-a-chip layers.
In organ-on-a-chip (OOC) processes, a cell culture membrane is placed in between two resealable glass slides to form two separate flow chambers. This allows two different flows, of either liquids or gases, on either side of the membrane. This dynamic microfluidic flow approach enables new and innovative ways to culture cells and tissues while offering precise and continuous control during the complete culturing process. The membrane layer together with a makes an organ-on-a-chip resealable flow cell.
The OOC middle layer is available in the following configurations:
|
SKU |
Pore size |
Membrane thickness [µm] |
Pore density [per cm2] |
| 11000738 | 0.45 | 12 | 2.00E+6 |
| 11002081 | 1 | 11 | 2.00E+6 |
| 11001206 | 3 | 9 | 8.00E+5 |
| 11001060 | 8 | 16 | 6.00E+4 |
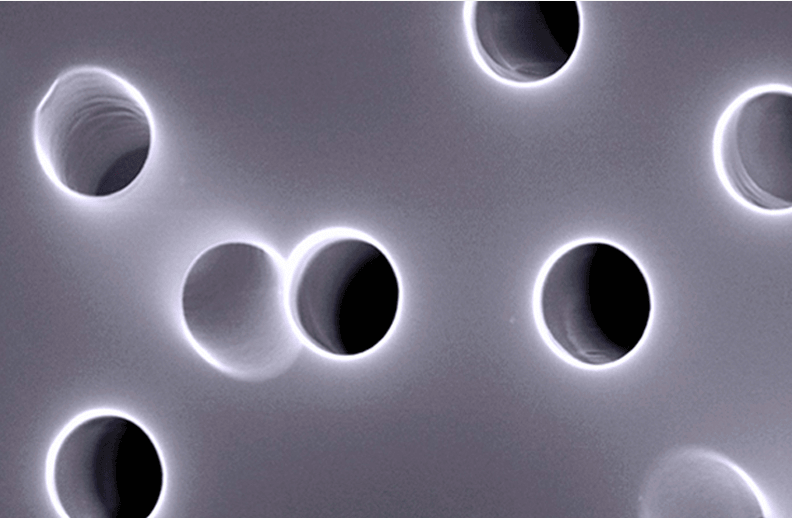
The pores are created using track-etching and have a random orientation:
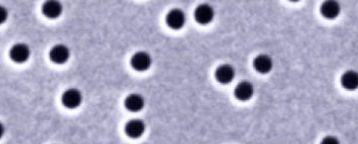
Using a small angle the random direction is shown:
| Unit of measurement | pack of 12 |
|---|---|
| Interface type | Topconnect |
| Carrier thickness | 0.4mm |
| Carrier material | Borosilicate glass |
| Membrane material | PET |
| Membrane appearance | Transparent |
| Membrane surface treatment | Cell culture treated |
| Icon | Label | Description | Type | Size | Download |
|---|---|---|---|---|---|
 | Organ-on-a-chip drawing | 118.4 KB | Download | ||
 | Organ-on-a-chip manual | Manual that helps to get started with OOC using the resealable platform. | 1.5 MB | Download | |
 | Compatibility with cell imaging systems | 153.1 KB | Download |